Copper Ii Sulfate Pentahydrate Dissolved Water Chemical Equation
You may need to predict some of the products or reactants a. The of such a pentahydrate is prepared in a.
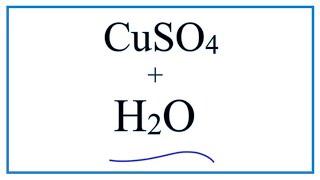
Equation For Cuso4 H2o Copper Ii Sulfate Water Youtube
Transfer the solid to a beaker.

. The second half is sulfate which you will need to memorize as SO4 with a charge of -2. Or if any of the following reactant substances CuSO4 copperii. Physical and Chemical Properties Molecular.
The white anhydrous copperII sulfate is then rehydrated and the blue colour returns. Web What is the chemical reaction equation if the method below is carried out. Web What type of chemical reaction is heating of copper sulfate.
Web Answer 1 of 2. Thus copper sulfate reacts with water in a 15 ratio and the product is copper sulfate pentahydrate. In this case you just need to observe to see if product substance CuSO4 copperii sulfate appearing at the end of the reaction.
The anhydrous Copper sulfate salt is pale green in color. Web The copper sulfate pentahydrate is an inorganic compound formed by the elements copper Cu sulfur S oxygen O and water H 2 OR. CuSO4 5H2OTo write the formula for Copper II sulfate pentahydrate w.
The combution of heptane produces carbon. Web Phenomenon after CuSO45H2O Copper sulfate pentahydrate This equation does not have any specific information about phenomenon. On the product side.
CuSO4o5H2O -- CuSO4 5H2O On the reactant side the copperIIsulfate is chemically bonded to the 5 water molecules. Web In this video well write the correct formula for Copper II sulfate pentahydrate. Its chemical formula is CuSO 4 5H 2 OR.
Web CopperII sulfate also known as cupric sulfate copper sulphate or archaically blue vitriol or vitriol of Cyprus is the chemical compound with the chemical formula CuSO 4This salt exists as a series of compounds that differ in their degree of hydrationThe anhydrous form is a pale green or gray-white powder whereas the. Contains copper II ions Cu 2 and sulfate SO 42-. It states the molecule is CuII so you know that Cu has a 2 charge.
Web Phenomenon after H2O water reacts with CuSO4 copperii sulfate This equation does not have any specific information about phenomenon. Condensing the vapour produced in a second test tube collects the water. First you need to determine how many ionic species make this compound what they are and what charge state theyre in.
Web The relevant chemical reaction is. Add 3g copperII sulfate pentahydrate to 25 cm3 water and heat to dissolve. Web It exothermically dissolves in water to give the aquo complex ceCuH2O62 which has octahedral molecular geometry.
Web Copper ii sulfate pentahydrate formula decomposition hydrate what is it and how to calculate the percent of water in do you know when hydrated evaporates socratic worksheet stoichiometric calculations 2 xtra chegg com thermal types chemical reactions wikispacespetyapisanscienceaq 3 experiment pdf document 7 hydrates name report for il. Or if any of the following reactant substances CuSO45H2O Copper sulfate pentahydrate. CopperII sulfate also known as copper sulphate are the inorganic compounds with the chemical formula Cu SO 4 H 2 O x where x can range from 0 to 5The pentahydrate x 5 is the most common form.
Students remove the water of crystallisation from hydrated copperII sulfate by heating. Add this solution to the CuSO45H2O solution. Cool the solution and filter using a Buchner funnel.
In nature it is found forming the mineral chalcantite or calcantite also called chalclase or calclasse. Web b Copper II sulfate pentahydrate dissolves in aqueous ammonium chromate to form solid Copper II chromate c Barium sulfide dissolves in concetrated nitric acid to produce dihydrogen sulfide. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands.
In this case you just need to observe to see if product substance CuSO45H2O Copper sulfate pentahydrate appearing at the end of the reaction. The ceCuIIH2O4 centers are interconnected by sulfate anions. Copper ii sulfate solubility.
Web Science Chemistry QA Library copper II sulfate pentahydrate is a blue compound with the chemical formula It is a salt because it contains water molecules it is ionic because it consists of an and a The copper ions are monatomic where the sulfate ion is A standard solution of copper II sulfate flask. Write and balance chemical equations. Web The equation for the dehydration of copperIIsulfate is.
Web The nitrates chlorates and acetates of all metals are soluble in water. Dissolve 25g glycine in hot 12cm3 2M NaOH solution.

How To Balance Cuso4 5h2o Cuso4 H2o Youtube
Chemistry Hess S Law Copper Sulfate Solution

Equation For Cuso4 H2o Copper Ii Sulfate Water Youtube

Balancing The Chemical Equation Cuso4 5h2o Cuso4 H2o Youtube
0 Response to "Copper Ii Sulfate Pentahydrate Dissolved Water Chemical Equation"
Post a Comment